AVITA Medical is focused on transforming lives by providing innovative ways of healing skin.
Regenerative Medicine for Skin Restoration
AVITA Medical is focused on transforming lives by providing innovative ways of healing skin.
Regenerative Medicine for Skin Restoration
AVITA Medical is focused on transforming lives by providing innovative ways of healing skin.
Regenerative Medicine for Skin Restoration
AVITA Medical® is a regenerative medicine company leading the development and commercialization of devices and autologous cellular therapies for skin restoration. Our FDA-approved RECELL® System technology platform treats patients with thermal burn wounds and full-thickness skin defects and is used for re-pigmentation of stable depigmented vitiligo lesions by harnessing the regenerative properties of a patient’s own skin to create Spray-On Skin™ cells.
We’re Changing Lives for the Better.

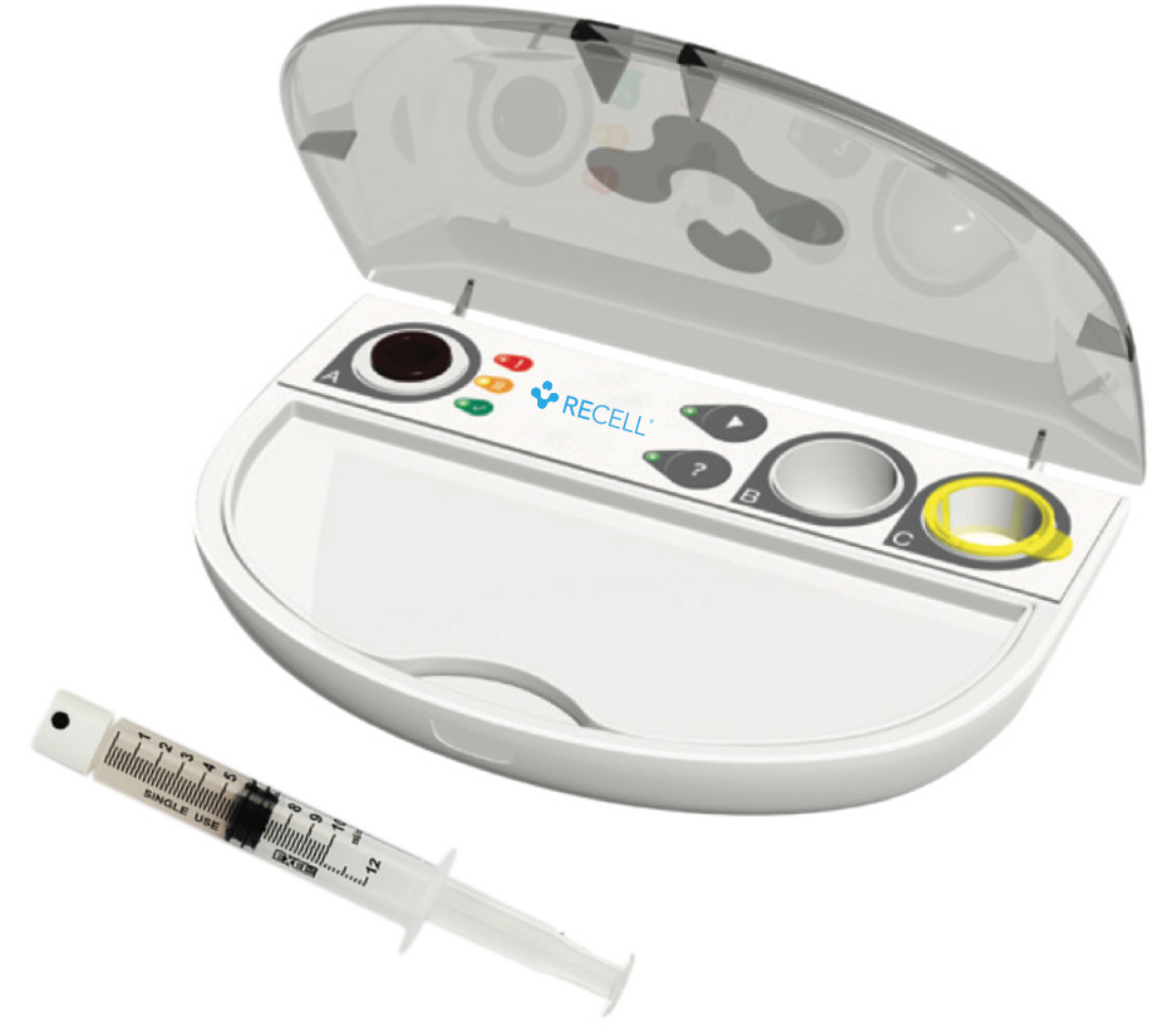
The RECELL® System is a device that enables healthcare professionals to produce a suspension of Spray-On Skin™ Cells using a small sample of the patient’s own skin for the treatment of thermal burn wounds and full-thickness skin defects. This is prepared and applied at the point of care in as little as 30 minutes.
Focused Pipeline
with Strong
Growth Potential
We are currently exploring the potential of our novel technology platform to harness the regenerative
properties of a patient’s own skin across
a number of skin-related indications.
properties of a patient’s own skin across
a number of skin-related indications.
Join Our Team
We believe a great culture attracts and retains quality talent. That is why we have intentionally created, and continue to nurture, a high-performance, inspired culture with a collaborative work environment where we are all passionate about making a difference in patient’s lives.

